2. Proton gradient driven Membrane Transport
Bacteria also use proton gradients generated during electron transport to drive active transport.
The membrane transport proteins responsible for this process lack special periplasmic solute binding proteins.
The lactose permease of E. coli is a
well-studied example. The
permease is a single protein having a molecular weight of about 30,000. It transports
a lactose molecule inward as a proton simultaneously enters the cell (a higher concentration of protons is maintained outside the
membrane by electron transport chain activity). Such linked transport of two substances in the same direction is called symport.
Here, energy stored as a proton gradient drives solute transport. The
binding of a proton to the transport protein increases
its affinity for the solute and
transports it.
E. coli also uses proton symport to take up amino acids and organic
acids like succinate and malate.
Active
Transport Using Proton and Sodium Gradients-
A proton gradient also can power active transport indirectly, often through the formation of a sodium ion gradient.
For example, an E. coli sodium transport system pumps sodium outward in response to the inward movement of protons. Such linked transport in which the transported substances move in opposite directions is termed antiport.
The sodium gradient generated by this proton antiport system then drives the uptake of sugars and amino acids. A sodium ion could attach to a carrier protein, causing it to change shape. The carrier would then bind the sugar or amino acid tightly and orient its binding sites toward the cell interior. Because of the low intracellular sodium concentration, the sodium ion would dissociate from the carrier, and the other molecule would follow.
E. coli transport proteins carry the sugar melibiose and the amino acid
glutamate when sodium simultaneously moves inward, this is Sodium symport or cotransport..
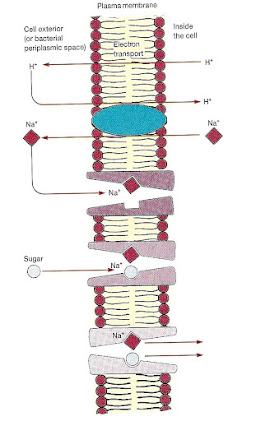
1 . Protons (H+) are pumped to the outside of the plasma membrane during electron transport.
2. The proton gradient drives sodium ion (Na+) expulsion by an
antiport mechanism.
3. The shape of the solute binding site changes, and it binds the solute (e.g., a sugar or amino acid). Sodium binds to the carrier protein complex.
4. The carrier’s conformation then alters so that sodium is released on the inside of the membrane. This is followed by solute dissociation from the carrier (a symport mechanism).
When there are several transport systems for the same substance, the systems differ in such properties as their energy source, their affinity for the solute transported, and the nature of their regulation. This diversity gives the organism an added competitive advantage in a variable environment.




No comments:
Post a Comment